Understanding
CIP (Clean-in-Place) Systems in Water Treatment
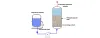
Clean-in-Place(CIP) systems are essential in maintaining the hygiene and efficiency of water
treatment and processing equipment. CIP technology allows for the automated
cleaning of machinery and pipelines without requiring disassembly, ensuring
consistent cleanliness and operational efficiency. This blog explores the
fundamentals of CIP systems, their components, and their importance in
maintaining optimal performance in water treatment facilities.
What is
CIP (Clean-in-Place)?
CIP is a
method used to clean the interior surfaces of pipes, vessels, equipment, and
other components of a water treatment system without needing to dismantle the
equipment. This process is essential for ensuring that the equipment remains
free from contaminants and operates efficiently, contributing to the overall
effectiveness of the water treatment process.
Key
Components of CIP Systems
- Importance
of CIP Systems
- CIP systems ensure that
equipment is consistently cleaned, preventing the buildup of contaminants
and residues that could affect water quality and system performance.
- By automating the cleaning
process, CIP systems reduce downtime and labor costs associated with
manual cleaning. This leads to more efficient operation and increased
productivity.
- Regular and effective cleaning
helps to extend the lifespan of equipment by preventing corrosion,
fouling, and other issues that can arise from inadequate cleaning.
- CIP systems help facilities
meet hygiene and safety regulations by providing a reliable and
documented cleaning process. This is particularly important in industries
where cleanliness is critical, such as food and beverage processing.
How CIP
Systems Work
- The cleaning solutions are
prepared and heated to the required temperature. The equipment is then
set up to allow for the circulation of the cleaning solutions.
- Cleaning solutions are pumped
through the equipment, ensuring that all surfaces come into contact with
the solution. The circulation process typically includes multiple stages,
such as pre-rinsing, cleaning, and post-rinsing.
- After the cleaning solutions
have been circulated, they are drained from the system. The equipment is
then rinsed with water to remove any residual cleaning chemicals.
- The CIP process is validated
through testing and inspection to ensure that the equipment has been
adequately cleaned and is ready for operation.
ConclusionCIP systems
are integral to maintaining the cleanliness and efficiency of water treatment
and processing equipment. By automating the cleaning process, CIP systems
enhance hygiene, improve operational efficiency, and extend equipment life. At
Aquafit Technology, we provide advanced CIP solutions tailored to meet the
specific needs of your water treatment operations, ensuring optimal performance
and compliance with industry standards.
CIP
Media: Essential for Effective Clean-in-Place Systems
Clean-in-Place
(CIP) systems are vital for maintaining the cleanliness and efficiency of water
treatment and processing equipment. CIP media are specialized materials used
within these systems to enhance the cleaning process. By improving the
effectiveness of the cleaning solutions, CIP media ensure that equipment
remains hygienic and operational. This blog explores the types, importance, and
applications of CIP media in water treatment systems.
What is
CIP Media?
CIP media
are materials designed to assist in the cleaning of equipment within CIP
systems. These media enhance the effectiveness of cleaning solutions by
improving their contact with surfaces, facilitating the removal of
contaminants, and ensuring thorough cleaning.
Types of
CIP Media
- Filter Media:
- Polypropylene filters are
used to remove particulate matter from CIP solutions, preventing
clogging and ensuring the cleanliness of the solutions. They are
resistant to a wide range of chemicals and temperatures.
- Stainless steel filters are
durable and can handle high temperatures and corrosive chemicals. They
are used in CIP systems where high filtration efficiency is required.
- Scrubbing Media:
- Polyester scrubbers are used
to physically scrub surfaces within the equipment. They help in
dislodging and removing residues and contaminants that may be difficult
to remove with cleaning solutions alone.
- Nylon brushes are used in
conjunction with cleaning solutions to scrub and clean surfaces. They
are effective in reaching into crevices and removing stubborn residues.
- Adsorption Media:
- Activated carbon is used to
adsorb organic contaminants and impurities from CIP solutions. It
enhances the effectiveness of the cleaning process by removing residual
organic matter.
- Ion exchange resins are used
to remove specific contaminants from cleaning solutions. They can be
tailored to target particular impurities, improving overall cleaning
efficiency.
- Chemical Media:
- Alkaline cleaning media, such
as sodium hydroxide, are used to break down organic residues and fats.
They are effective in cleaning heavily soiled equipment.
- Acidic cleaning media, such
as citric acid or phosphoric acid, are used to remove inorganic scale
and mineral deposits. They are essential for maintaining equipment that
is prone to scaling.
Importance
of CIP Media
- Enhanced Cleaning Efficiency:
- CIP media improve the
effectiveness of cleaning solutions by aiding in the removal of
contaminants and residues. This ensures that equipment is thoroughly
cleaned and maintained.
- By ensuring thorough cleaning,
CIP media help to prevent equipment damage and extend its lifespan. This
reduces the need for repairs and replacements.
- Effective cleaning with CIP
media reduces the frequency and duration of equipment downtime for
cleaning. This leads to increased operational efficiency and
productivity.
- Compliance with Standards:
- CIP media help facilities meet
hygiene and safety standards by ensuring that cleaning processes are effective
and documented. This is crucial for industries with strict cleanliness
requirements.
How CIP
Media Work
- Integration:
- CIP media are integrated into
the CIP system, where they come into contact with cleaning solutions and
equipment surfaces. Their properties and materials are chosen based on
the specific cleaning needs.
- Interaction with Cleaning
Solutions:
- The media enhance the action
of cleaning solutions by improving their contact with surfaces,
facilitating the removal of contaminants, and ensuring that solutions
reach all areas of the equipment.
- Maintenance and Replacement:
- Regular maintenance and
replacement of CIP media are essential to ensure their continued
effectiveness. This includes cleaning or replacing filters, scrubbing
media, and adsorption materials as needed.
ConclusionCIP media
play a crucial role in the effectiveness and efficiency of Clean-in-Place
systems. By enhancing the cleaning process, these media ensure that equipment
remains hygienic, extends its operational life, and reduces downtime. At
Aquafit Technology, we offer a range of high-quality CIP media tailored to meet
the specific needs of your water treatment system, ensuring optimal performance
and compliance with industry standards.
How to
Wash Membranes Using CIP (Clean-in-Place) Systems
Membranes
used in water treatment systems, such as Reverse Osmosis (RO) and
Ultrafiltration (UF), require regular cleaning to maintain their performance
and extend their lifespan. Clean-in-Place (CIP) systems offer an efficient way
to wash these membranes without disassembling the system. This blog explores
the steps and best practices for washing membranes using CIP, ensuring optimal
membrane performance and longevity.
What is
CIP (Clean-in-Place)?
CIP is a
process used to clean equipment and systems without dismantling them. In the
context of membrane systems, CIP involves circulating cleaning solutions
through the membrane modules to remove fouling and contaminants that may affect
performance.
Steps to
Wash Membranes Using CIP
- Preparation:
- Prepare Cleaning Solutions:
- Circulate Cleaning Solutions:
- Best
Practices for CIP Membrane Cleaning
- ConclusionCIP systems
provide an effective and efficient method for washing membranes in water
treatment systems. By following the steps and best practices outlined above,
you can ensure thorough cleaning, maintain optimal membrane performance, and
extend the lifespan of your equipment. At Aquafit Technology, we offer advanced
CIP solutions and support to help you achieve the best results in membrane
cleaning and water treatment.
Understanding
OBR (Ozone-Biological Reactor) in Water Treatment
The Ozone-Biological
Reactor (OBR) is an advanced water treatment technology that integrates ozone
treatment with biological processes to achieve superior water quality. OBR
systems are designed to address various water treatment challenges, including
contaminant removal, disinfection, and pollutant degradation. This blog
explores the principles of OBR technology, its components, benefits, and
applications in water treatment.
What is
OBR (Ozone-Biological Reactor)?
An
Ozone-Biological Reactor (OBR) combines ozone oxidation with biological
treatment methods in a single system. The OBR process involves the use of ozone
gas to oxidize and break down contaminants, followed by biological treatment to
further degrade pollutants and enhance water quality. This integrated approach
offers a comprehensive solution for effective water treatment.
Key
Components of OBR Systems
- Ozone Generation Unit:
- Control and Monitoring Systems:
- Benefits
of OBR Technology
- Enhanced Contaminant Removal:
- Applications
of OBR Technology
- Drinking Water Treatment:
- OBR systems are used to
produce high-quality drinking water by removing contaminants and ensuring
safety.
- In municipal and industrial
wastewater treatment, OBR systems help to reduce pollutants and improve
effluent quality before discharge or reuse.
- OBR technology is used in
various industrial processes where high-quality water is required, such
as in food and beverage production, pharmaceuticals, and electronics
manufacturing.
Conclusion
Ozone-Biological
Reactor (OBR) technology offers a robust and effective solution for advanced
water treatment. By combining the powerful oxidizing effects of ozone with
biological degradation processes, OBR systems achieve superior contaminant
removal and water quality improvement. At Aquafit Technology, we provide
state-of-the-art OBR solutions tailored to meet the specific needs of your
water treatment applications, ensuring optimal performance and compliance with
quality standards.
Resin
Regeneration: Essential for Maintaining Ion Exchange Efficiency
Resin
regeneration is a crucial process in the maintenance of ion exchange systems
used in water treatment. Over time, ion exchange resins, which are used to
remove contaminants from water, become saturated with ions and lose their
effectiveness. Regeneration is the process of restoring these resins to their
original state, ensuring their continued efficiency and longevity. This blog
explores the resin regeneration process, its importance, methods, and best
practices.
What is
Resin Regeneration?
Resin
regeneration refers to the chemical and physical processes used to restore ion
exchange resins to their optimal performance levels after they have been
exhausted by ion exchange reactions. During regular operation, resins capture
and hold onto undesirable ions from water, gradually becoming less effective.
Regeneration recharges the resin with the necessary ions to continue effective
water treatment.
Importance
of Resin Regeneration
- Maintains System Efficiency:
- Regeneration ensures that ion
exchange resins continue to operate efficiently by restoring their
capacity to exchange ions. This maintains the overall performance of the
water treatment system.
- Extends Resin Life:
- Proper regeneration extends
the lifespan of ion exchange resins, reducing the need for frequent
replacement and associated costs.
- Reduces Operational Costs:
- By keeping resins effective and
extending their operational life, regeneration minimizes the need for
resin replacement and helps control overall water treatment costs.
- Ensures Water Quality:
- Effective resin regeneration
ensures consistent water quality by maintaining the performance of ion
exchange systems, which is crucial for meeting regulatory standards and
achieving desired water treatment outcomes.
Methods
of Resin Regeneration
- Chemical Regeneration:
- Physical Regeneration:
- Electrochemical Regeneration:
- Best
Practices for Resin Regeneration
- Adhere to the resin
manufacturer's recommendations for regeneration procedures, including the
type and concentration of regenerant chemicals, flow rates, and contact
times.
- Regularly check the condition
of the resin and the effectiveness of the regeneration process. Adjust
regeneration parameters as needed based on resin performance and water
quality.
- Ensure that the equipment used
for regeneration, such as regenerant tanks, pumps, and valves, is
well-maintained and compatible with the resin and chemicals used.
- Use the appropriate amount of
regenerant to avoid excessive use and potential resin damage. Overuse or
underuse of chemicals can affect the effectiveness and lifespan of the
resin.
- Dispose of spent regenerant
solutions and waste materials according to environmental regulations to
minimize environmental impact.
ConclusionResin
regeneration is a vital process for maintaining the efficiency and longevity of
ion exchange systems in water treatment. By restoring ion exchange resins to
their optimal condition, regeneration ensures consistent water quality, extends
resin life, and reduces operational costs. At Aquafit Technology, we provide
expert solutions and support for resin regeneration, helping you achieve
optimal performance and reliability in your water treatment systems.
Resin
Calculation: Essential for Efficient Ion Exchange Systems
Proper
resin calculation is crucial for the effective design and operation of ion
exchange systems used in water treatment. Accurate calculations ensure that the
right amount of resin is used to meet the desired treatment capacity and
performance requirements. This blog will guide you through the process of
calculating the required resin quantity, taking into account factors like ion
exchange capacity, water quality, and system design.
Key
Factors in Resin Calculation
- Water Quality Parameters:
- Resin Characteristics:
- System Design Parameters:
- Steps to
Calculate Resin Requirements
- Calculate the Contaminant Load:
- Calculate Resin Capacity
Required:
- Determine Resin Bed Size:
- Consider Regeneration and
Service Life:
- 5.
Total Contaminant Load:
1.Total
Contaminant Load:
Total Contaminant Load=
Concentration × Flow Rate
=50mg/L×10,000L/day
=500,000mg/day
2.Convert
to Equivalents:

3.Calculate
Required Resin Volume:

If the
resin capacity is 0.5 eq/L, adjust accordingly.
Accurate
resin calculation is vital for the effective design and operation of ion
exchange systems. By considering water quality, resin characteristics, and
system design parameters, you can determine the appropriate resin volume and
bed size required for optimal performance. At Aquafit Technology, we provide
expert support and advanced solutions for resin calculations and system design,
ensuring your water treatment systems operate efficiently and effectively.
Antiscalant
Dosing Calculation: Essential for Effective RO System Performance
Antiscalants
are crucial for preventing scaling and fouling in Reverse Osmosis (RO) systems,
which can otherwise lead to reduced efficiency and frequent maintenance issues.
Accurate dosing of antiscalants ensures optimal performance of the RO system,
preventing scale formation and extending membrane life. This blog provides a
detailed guide on how to calculate the appropriate dosing of antiscalants for
RO systems.
Factors
Influencing Antiscalant Dosing
- Water Quality:
- RO System Specifications:
- Antiscalant Properties:
- Steps
for Antiscalant Dosing Calculation
- Determine Scaling Potential:
- Select Antiscalant Product:
-
- Calculate
Required Dosage:

Adjust
for Concentration:

- .Determine
Injection Rate:
Injection
Flow Rate:

Consider an
RO system with a feed flow rate of 100 m³/day, and you are using an antiscalant
with a recommended dosing rate of 2 mg/L.
1.Calculate
Daily Antiscalant Dose:
Daily Dose=
2mg/L ×100m³/day
=200mg/day
2.Convert
to Volume:
If the
antiscalant solution has a concentration of 10 g/L:

3.Determine
Injection Rate:

Conclusion
Accurate
dosing of antiscalants is vital for preventing scaling and fouling in RO
systems, ensuring efficient operation and prolonging membrane life. By
considering factors such as water quality, RO system specifications, and antiscalant
properties, you can calculate the appropriate dosage and injection rate. At
Aquafit Technology, we provide expert guidance and high-quality antiscalant
solutions to help you maintain optimal performance in your water treatment
systems.
Chlorination
Media and Dosing: Ensuring Safe and Effective Water Disinfection
Chlorination
is a widely used method for disinfecting water, ensuring it is safe for
drinking and other applications. The process involves adding chlorine or
chlorine compounds to water to eliminate harmful bacteria, viruses, and other
microorganisms. This blog explores the types of chlorination media, the
importance of proper dosing, and how Aquafit Technology provides solutions for
effective water disinfection.
Types of
Chlorination Media
- Liquid Chlorine (Sodium
Hypochlorite):
- Importance
of Proper Chlorination Dosing
- Steps
for Chlorination Dosing Calculation
- Determine Water Flow Rate:
- Measure the volume of water to
be treated, typically in liters per second (L/s) or gallons per minute
(GPM).
- Calculate Required Chlorine
Dose:
- The dose is typically
expressed in milligrams per liter (mg/L). The required dose depends on
the quality of the raw water, the target residual chlorine level, and the
specific chlorination media used.
- Adjust for Chlorine Demand:
- Consider the chlorine demand
of the water, which is the amount of chlorine that reacts with organic
and inorganic substances before a residual is maintained.
- Set Dosing Rate:
- Use a dosing pump to
accurately inject the calculated amount of chlorine into the water
stream. The dosing rate can be adjusted based on real-time monitoring of
chlorine levels.
Conclusion
Chlorination
is a critical step in water treatment, ensuring that water is safe for human
consumption and industrial use. By understanding the types of chlorination
media and calculating the correct dosage, water treatment professionals can
optimize the disinfection process. At Aquafit Technology, we provide a range of
chlorination solutions tailored to your specific needs, ensuring effective and
safe water treatment.
Membrane
Anti-Blockage Media: Ensuring Efficient Water Treatment
In water
treatment plants, membrane technologies such as reverse osmosis (RO),
ultrafiltration (UF), and nanofiltration (NF) are widely used to purify water
by removing contaminants. However, these membranes are prone to fouling or
blockage, which can reduce their efficiency and lifespan. Membrane
anti-blockage media are specialized chemicals and materials designed to prevent
or minimize membrane fouling, ensuring the smooth operation of water treatment
systems.
Types of
Membrane Anti-Blockage Media
- Antiscalants:
- Detergents and Surfactants:
- Importance
of Membrane Anti-Blockage Media
- Effect
of Membrane Blockage
- ConclusionMembrane
anti-blockage media play a crucial role in ensuring the efficient operation of
water treatment plants that rely on membrane technology. By preventing fouling
and blockage, these media help maintain system efficiency, reduce operational
costs, and prolong the life of membranes. Aquafit Technology offers a range of
high-quality membrane anti-blockage solutions tailored to meet the specific
needs of your water treatment system. With our expertise, we ensure that your
membranes remain clean, efficient, and long-lasting.
Vessel Multi-Media
Washing Solution: Essential for Efficient Water Filtration
In water
treatment systems, multi-media filters are essential for removing suspended
solids, turbidity, and other impurities from water. These filters contain
layers of different media such as sand, anthracite, and gravel, which work
together to trap particles as water passes through them. Over time, these media
can become clogged with debris, reducing the filter's efficiency. A vessel
multi-media washing solution is vital for maintaining the effectiveness of
these filters, ensuring clean and safe water.
What is
Vessel Multi-Media Washing?
Vessel
multi-media washing involves the cleaning of the filter media within a
multi-media vessel to restore its filtration capacity. This process typically
includes backwashing and rinsing, where water or a cleaning solution is passed
through the filter in the opposite direction to remove trapped particles and
contaminants.
Importance
of Regular Washing
- How
Vessel Multi-Media Washing Works
- Benefits
of Vessel Multi-Media Washing
- ConclusionA vessel
multi-media washing solution is a crucial maintenance practice for any water
treatment system that uses multi-media filters. It ensures the continued
efficiency of the filtration process, prolongs the life of the media, and
guarantees high water quality. At Aquafit Technology, we provide comprehensive
solutions for maintaining and optimizing your water treatment systems,
including expert multi-media washing services. Our solutions ensure that your
filters operate at peak performance, delivering clean and safe water every
time.
Brine
Dosing Calculation: A Guide for Water Softening Systems
Brine
dosing is a critical process in water softening systems, where a solution of
salt (sodium chloride) is used to regenerate the ion exchange resins. These
resins, responsible for removing hardness-causing minerals like calcium and
magnesium, become saturated over time and require regeneration to maintain
their efficiency. Proper brine dosing ensures that the ion exchange process
continues effectively, delivering soft water for industrial, commercial, or
residential use.
Understanding
Brine Dosing
Brine
dosing refers to the controlled addition of a salt solution into the ion
exchange resin tank during the regeneration phase. The amount of brine used
must be carefully calculated to ensure the resin is fully regenerated without
wasting excess salt.
Steps
for Brine Dosing Calculation
o o
Formula:
Salt Dose(lbs)=Resin Volume(ft³)×Salt Dose Rate(lb/ft³)
Example: If you have 2 ft³ of resin and
use a salt dose rate of 8 lb/ft³:
2ft³×8lb/ft³=16lbs of salt
3.Calculate Brine Solution Volume:
A standard brine solution is about 10% sodium
chloride by weight. This means that 1 gallon of brine contains approximately
2.5 pounds of salt.
Formula:

Example:
With a required salt dose of 16 lbs:

4.Determine
the Brine Tank Size:
Ensure that
your brine tank can hold the calculated brine solution volume. The tank should
have enough capacity to accommodate both the brine solution and the salt bed,
allowing for proper mixing and dosing.
Adjust
Based on System Needs:Depending on the specific
requirements of your water softener system, you may need to adjust the
salt dose or brine concentration. Some systems may allow for more
efficient regeneration with lower salt doses, while others may require
higher doses for heavily saturated resins.
Effect
of Incorrect Brine Dosing
- Conclusion
Proper
brine dosing calculation is essential for maintaining the efficiency and
longevity of water softening systems. By accurately determining the required
salt dose and brine solution volume, you can ensure effective resin
regeneration, consistent soft water production, and optimal system performance.
Aquafit Technology provides expert guidance and solutions for all your water treatment
needs, including precise brine dosing and system maintenance.